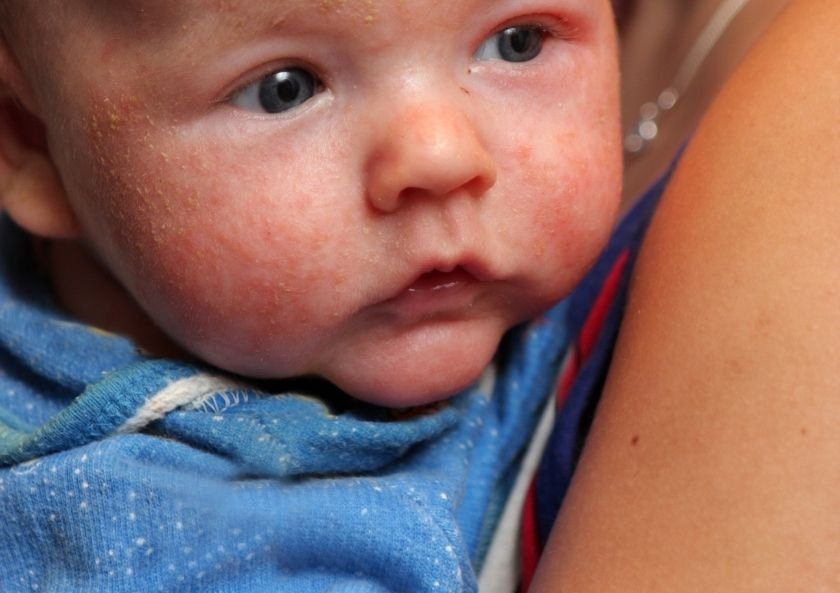
Dupilumab is a monoclonal antibody of human origin that binds and blocks the IL4 receptor alpha subunit, which is a common structure on IL-4 and IL-13 receptors. Blocking this receptor leads to the blocking of both IL-4 and IL-13-mediated signaling, and as a result, suppression of the TH2 inflammatory response, which has an important place in the pathogenesis of atopic dermatitis.
Who Is Dupilumab Used For?
Its use in the treatment of atopic dermatitis was approved by the FDA in March 2017 for use in moderate to severe adult atopic dermatitis cases where topical treatments have failed or are not recommended.
May 26, 2020 – The U.S. Food and Drug Administration (FDA) has approved Dupixent® (dupilumab) for children ages 6 to 11 years with moderate to severe atopic dermatitis whose disease is not adequately controlled with topical prescription treatments or when these treatments are not recommended. Dupixent is the only biologic drug approved for this patient population.
Approved in Turkey over the age of 12 yet.
Dupilumab is effective in 3 groups of diseases;
- Atopic dermatitis
- Asthma
- Chronic rhinosinusitis and nasal polyp
“We continue to study Dupixent in young children 6 months to 5 years of age with uncontrolled moderate to severe atopic dermatitis and children with uncontrolled, persistent asthma.
In addition, Dupixent is being studied for efficacy in other diseases caused by type 2 inflammation, including eosinophilic esophagitis, food and environmental allergies, chronic obstructive pulmonary disease, and other dermatological diseases. ”
What is Dupilumab’s Effectiveness?
Dupilumab is effective in the treatment of atopic dermatitis. It improves skin signs, symptoms, severity and quality of life. It has been reported that the complaints of itching improved by 75% with 12 weeks of treatment. The suppression of itching provides a significant increase in the quality of life. Suppression of itching; it also provides significant improvement in sleep patterns, depression, anxiety and quality of life.
What is Dupilumab Dosage and Administration?
Dupilumab for eczema is an applicable treatment method used in moderate and severe atopic dermatitis aged 12 years and over. This therapy is also effective in patients with moderate to severe asthma with the eosinophilic asthma phenotype and corticosteroid-dependent asthma. It was also found to be effective in patients with nasal polyps and rhinosinusitis that did not respond to treatment.
Since there is no difference between 300 mg per week and 300 mg in 2 weeks, the most effective dose recommended by the FDA is 300 mg every 2 weeks.
For children under 60 kg, it is recommended to start with 400 mg and administer 200 mg every 2 weeks.
In adults or patients over 60 kg, the first dose is 600 mg, followed by 300 mg every 2 weeks. It is administered as a subcutaneous injection. When the patient is given the necessary training, the patient can also apply it at home.
If the dose is missed, this dose is administered within the first 7 days. If it is forgotten more, 14th day is expected.
It can be applied with topical treatments or alone. If it is to be applied with topical treatment, calcineurin inhibitors are applied only to problem areas. Efficacy is increased with topical corticosteroids.
What Are the Side Effects of Dupilumab for Eczema?
Dupilumab is a safe drug. The most common side effects include exacerbation of the disease, nasopharyngitis, headache, injection reactions, conjunctivitis, blepharitis, oral herpes infection, keratitis, itching of the eye, dry eye and other herpes infections. Generally, itching and skin infections are not common. Conjunctivitis and keratitis regress when treatment is discontinued. It is generally less common. Herpes infections are usually mild and in the form of herpes labialis.
Injection reactions are seen in 7% of patients. Its incidence and severity are not dose dependent.
It does not harm liver and kidney functions. There may be a mild eosinophilia only at the 3rd month of treatment.
When Does the Effect of Dupilumab Treatment Start?
Dupilumab for eczema controls the symptoms of atopic dermatitis. Significant improvement in symptoms is seen in the first 4 weeks.